Pelvic Floor
Anatomy of the Pelvic Floor
The term pelvic floor typically refers to the bony structures, endopelvic fascia, and muscles that support the pelvic organs and contribute to the sphincter mechanism for both the urinary and intestinal tracts.
Because the pelvic floor is difficult to visualize, its components bear multiple names, and one segment of a muscle or fascial sheet may be distinctly labeled as if it were a separate structure. For the purposes of this discussion, the bony pelvis, endopelvic fascia, and pelvic muscles are described as functional units, and the individual structures frequently named and described in surgical texts are described separately only if they directly contribute to our current understanding of continence or serve as a surgical landmark or point of attachment for the various surgical procedures used to manage urinary incontinence.
- Female Pelvic Floor
The bony pelvis of the female is a ring of bones that can be described according to their relative positions on the face of a clock (Figure X1, B). The 6 o’clock position is occupied by the symphysis pubis, and the pubic bones occupy the regions between 3 and 5 o’clock and between 7 and 9 o’clock.
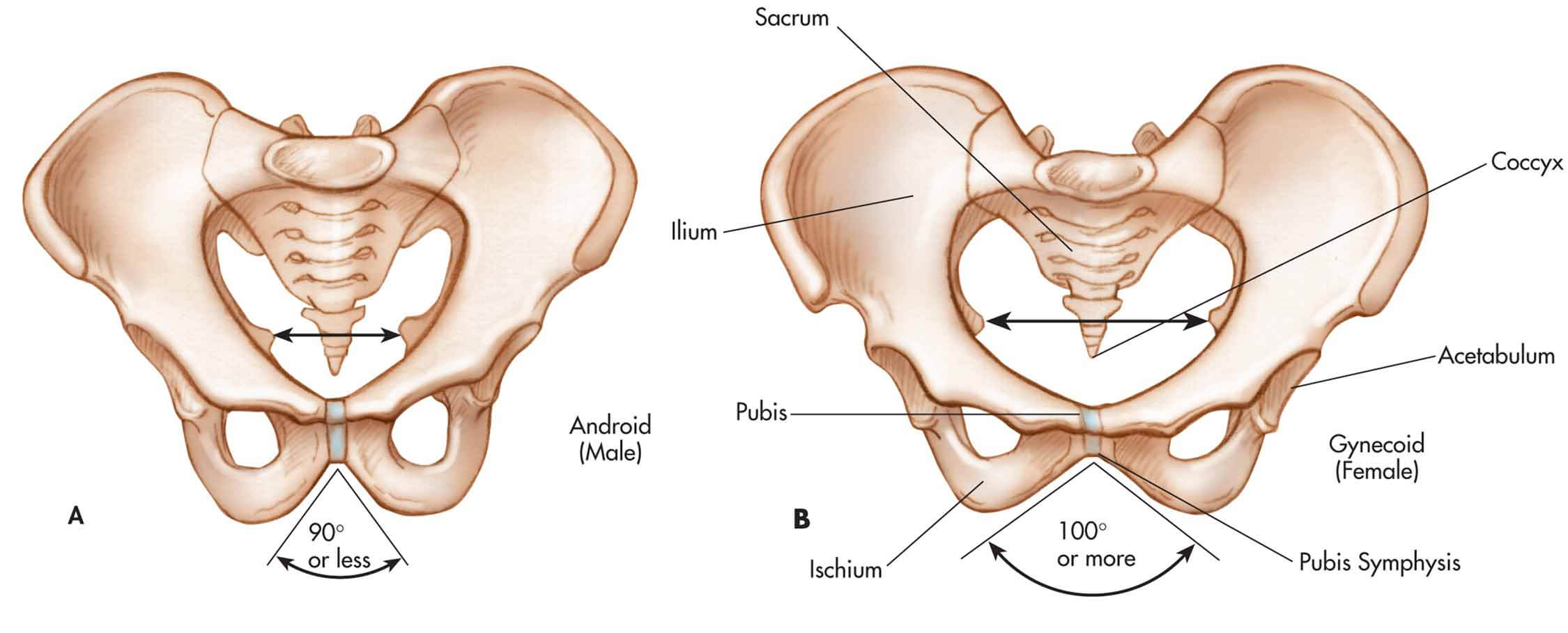
|
| Figure X1 | The bony pelvis of the male, A, and the female, B. |
The 12 o’clock position is occupied by the sacral bone, and the areas between 9 and 11 o’clock and between 1 and 3 o’clock are occupied by the pelvic side walls. (The pelvic side walls include the ischial spine, the sciatic foramen, and the sacral attachments.) All the muscles and endopelvic fascia of the female are anchored to the bony structures of the pelvis.
The endopelvic fascia of the female is continuous with the retroperitioneal fascia; it consists of fibromuscular tissue that contributes to the support of the pelvic organs, including the bladder base and the urethra. The endopelvic fascia can be subdivided into the endopelvic and levator portions, but for the purposes of this discussion the pelvic floor fascia is considered a single functional unit.
In addition to connective tissue, the endopelvic fascia also contains varying portions of smooth muscle and vascular elements; the specific “mix” of tissue types is dependent on the location and functional role of the specific structure.
Condensations of the endopelvic fascia are referred to as ligaments, although it is important to remember that these ligaments typically constitute only one aspect of a larger fascial plane. Collectively, the endopelvic fascia invaginates the pelvic viscera and provides a secondary source of support for these organs; the primary support structure for the pelvic organs is the levator ani muscle.
The upper vagina, cervix, and uterus are attached to the pelvic side walls by a relatively large sheet of endopelvic fascia, which originates at the greater sciatic foramen of the bony pelvis and inserts into the lateral walls of the cervix and the proximal third of the vagina. Condensations of this fascia are separately labeled as the cardinal and uterosacral ligaments, although these two structures are anatomically contiguous. Downward reflections of this fascial sheet support the middle portion of the vagina and attach to the pelvic side walls.
The endopelvic fascia (Figure X2) also forms a hammock that stretches and supports the vagina between the side walls of the pelvis; this hammock helps to prevent the bladder from prolapsing into the potential space of the vaginal vault. Posterior fascial structures extend this support and form a horizontal sheet that supports the rectum and prevents it from prolapsing forward into the potential space of the vagina.
These sheets of connective tissue are often labeled the “pubocervical” and “rectovaginal” fascia. Again, it is important to remember that they represent individual aspects of a larger, functionally continuous fascial sheet that provides support to the cervix, vagina, and upper uterus and indirect support to the bladder and rectum.
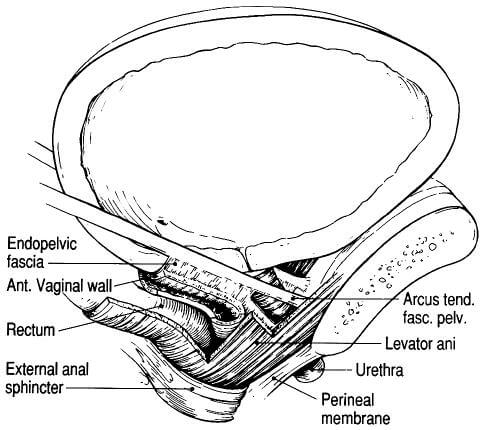
|
| Figure X2 | Schema of endopelvic structures in the female |
| Table X1 | Condensations of the Female Endopelvic Fascia | |
|---|---|
| Ligament | Description |
| Pubourethral ligaments | Condensations of endopelvic fascia that bridge lower, inner surface of symphysis pubis and middle of urethra |
| Urethropelvic ligaments | Endopelvic fascia providing support for bladder neck and proximal urethra |
| Vesicopelvic ligaments | Fascia condensations that attach to pelvic side walls providing lateral support to bladder base and pelvic side wall; loss of this fascial support creates a paravaginal wall defect |
| Cooper’s ligament | Condensation of connective tissue at the top of the crural arch from the inferior iliac to the pubic bone; provides support and structure for the floor of the inguinal canal |
| Broad ligament | Triangular fold of the peritoneum; supports uterus and fallopian tube |
| Round ligament | Passes from superior lateral angle of the uterus to the internal inguinal ring and supports uterus and fallopian tube |
| Uterosacral ligament (sacrouterine ligament, or cardinal-uterosacral complex) | Two short cords that pass from cervix toward sacrum; holds the cervix upward and backward and provides forward slant of uterus |
| Mackenrodt’s ligament (cardinal ligament) | Condensation of uterosacral fascial complex; contributes to support of upper vagina, cervix, and uterus |
| Sacrospinous ligaments | Attaches ischial spine to lateral aspect of sacrum and coccyx; used for repair of severe uterine prolapsed |
| Arcus tendineus fasciae pelvis/”white line” | Linear condensation extending from the pubic tone to the ischial spine; acts as important surgical landmark |
Specific fascial ligaments or condensations of larger fascial tissue planes are significant to any discussion of continence because they are used to create compensatory support during surgical correction of urethral hypermobility and pelvic organ prolapse Opens in new window associated with stress urinary incontinence. Table X1 summarizes specific areas of the endopelvic fascia that form important surgical landmarks and their functional significance to continence management.
The primary supportive muscle of the female pelvic floor is the levator ani. In the standing woman, the levator ani provides a horizontal plane of support for the bladder base, vagina, and adjacent pelvic organs. The levator also contributes to urethral sphincter function, particularly during periods of physical exertion.
As is true of the endopelvic fascia, various descriptions of the levator ani have led to confusion concerning its anatomic description; a review of 256 studies of the levator muscle in humans consistently revealed five pairs of origin and insertion points carrying as many as 16 labels in women alone.
Despite the variety of labels, the levator ani in female humans can be described according to these five pairs of origins and insertions, most commonly described as:
- puboperineal,
- pubovaginal,
- pubococcygeal (frequently called pubovisceral and puboanal),
- puborectal, and
- iliococcygeal.
Two portions are thought to play the most direct role in maintaining urinary continence, the pubococcygeal (pubovisceral or puboanal) and the ileococcygeus (Color Plate 1).
- The first portion consists of U-shaped muscle fibers that arise from the pubic bones, attach to the lateral walls of the vagina and rectum, and extend around the posterior portion of the rectum. This portion of the levator ani is called the “pubococcygeus,” or the “pubovisceral” muscle.
- The second portion of the levator ani is the relatively thin ileococcygeus muscle that originates from the pelvis behind the rectum.
Although five origin and insertion pairs for the levator ani have been identified (and assigned a wide variety of names), the continence nurse should remember that it comprises a single functional unit, particularly when viewed from the perspective of pelvic muscle rehabilitation.
- Male Pelvic Floor
The pelvic floor in the male, as in the female, is composed of the bony pelvis, endopelvic fascia, and pelvic muscles. The bony pelvis of the male is comparable to the female pelvis in many aspects.
The anterior portion of the male pelvis is formed by the symphysis pubis and pubic bones, the side walls are comparable, and the posterior surface comprises the coccyx and sacrum and their bony attachments. However, the pelvic side walls of the male have a steeper angle than the female’s, and the pelvic inlet is narrower than that of the female and is shaped more like a heart rather than the circular shape of the female inlet (Figure X1, A).
The pelvic fascia in the male is analogous to the pelvic fascia of the female in several ways: it is continuous with the retroperitoneal fascia, it can be divided into the endopelvic (abdominal) fascia and the levator ani fascia, and individual segments have been given specific names. However, it functions as a single unit, and for the purpose of this discussion, the term pelvic fascia is used to describe those elements that serve as a secondary supportive structure for the pelvic viscera in the male.
The puboprostatic fascia is the section that extends from the inner aspects of the symphysis pubis and pubic bones to the junction of the prostatic and membranous urethra; condensations of this fascia are known as the puboprostatic ligaments.
The puboprostatic fascia provides lateral support for the bladder base and urethra and inserts into the ischial spines of the male’s pelvic side walls. The pelvic fascia is located posteriorly to the ischial spines in the male; this segment extends from the rectum to the pelvic side walls, thus providing posterior support and separating the urethra, bladder base, seminal vesicles, and prostate gland from the rectum.
The anterior rectal wall, prostate gland, and seminal vesicles are separated by a thin layer of pelvic connective tissue called Denonvilliers’ fascia; this fascial sheet covers the posterior aspect of the prostate gland and then extends above the prostatic base to partially enfold the posterior aspect of the seminal vesicles but thins significantly as it approaches the membranous urethra (Table X2).
As in the female, primary support for the bladder base and urethra is provided by the levator ani muscle.
Similar to the female, two portions of the levator ani muscle. Similar to the female, two portions of the levator ani are considered most important to a discussion of continence, the U-shaped pubococcygeus (pubovisceral) and the ileococcygeus. In contrast to the female, the levator ani in the male is generally thicker and narrower, and it lacks a vaginal opening.
| Table X2 | Condensations of the Male Endopelvic Fascia | |
|---|---|
| Ligament | Functional Significance |
| Puboprostatic ligament | Attached to back of pelvic bone and to visceral layer of pelvic fascia on prostate, providing support for the prostate gland and bladder base |
| Denonvilliers’s fascia | Covers and fuses with pelvic endopelvic fascia covering the prostate; actually a reflection of peritoneal connective tissue |
| Arcus tendineus/“white line” | Linear condensation extending from the pubic bone to the ischial spine; indicates the origin of the levator ani |
Microscopic Anatomy of the Pelvic Floor
The endopelvic fascia is primarily composed of the connective tissues collagen and elastin. However, it may also contain vascular elements, neural elements, and smooth muscle, depending on its location in the pelvis and its functional role. For example, the cardinal ligaments contain vascular elements as well as connective tissue, whereas the perirectal fascia contains few vascular elements and more collagen.
Similarly, the pubovesical fascia contains smooth muscle, which is postulated to interact with muscular elements of the urethra to promote urethral closure during bladder filling and urethral funneling during micturition.
Thus, the endopelvic fascia provides a dynamic rather than a static source of support for the bladder base and urethra. This flexibility is an essential element of support for the varied functions of the pelvic floor, which include storage and elimination of urine and stool as well as labor and delivery in the female.
The microscopic anatomy of the levator ani muscle also provides important clues to its function. The levator ani muscle consists of skeletal muscle fibers that are innervated by branches of the pudendal nerve. As in all skeletal muscles, the functional element of the levator ani is the motor unit.
A motor unit consists of an anterior horn cell (neuron), its axon, a neuromuscular junction, and the muscle fibers; the neuromuscular junction provides “connection” and “communication” between the nervous system and the muscle fibers. Skeletal muscles can be divided into slow-twitch and fast-twitch fibers.
Type 1 (slow-twitch) fibers are adapted to maintain muscle tone over prolonged periods of time, whereas Type 2 (fast-twitch) fibers are physiologically designed to provide the rapid contraction required for sudden physical exertion. The levator ani is composed of approximately 70% Type 1 fibers and 30% Type 2 fibers.
The preponderance of slow-twitch fibers allows the muscle to provide sustained support for an individual in the upright (standing) position, whereas the fast-twitch fibers provide rapid contraction of the periurethral and perianal fibers whenever there is a need to increase sphincter tone to offset a sudden increase in abdominal force (as when a person coughs, sneezes, or lifts a heavy object).
See also:
- Moore KN, Paul P: A historical review of selected nursing and medical literature on urinary incontinence between 1850 and 1976, J Wound Ostomy Continence Nurs 24:106-122, 1997.
- Cooper CS, Abousally CT, Austin JC, et al: Do public schools teach voiding dysfunction: Results of an elementary school teacher survey, J Urol 170(3):956-958, 2003.
- Fitzgerald ST, Palmer MH, Kirkland VL, Robinson L: The impact of urinary incontinence in working women: a study in a production facility, Womens Health 35(1):1-16, 2002.
- Steers WD: Physiology of the urinary bladder. In Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors: Campbell’s urology, ed 6, Philadelphia, 1992, WB Saunders.
- Steers WD: Physiology and pharmacology of the bladder and urethra. In Walsh PC, Retik AB, Vaughan ED, Wein EJ, editors: Campbell’s urology, ed 7, Philadelphia, 1998, WB Saunders.
- Gray ML: Geniourinary disorders, St. Louis, 1992, Mosby.
- Dixon J, Gosling J: Structure and innervations in the human. In Torrens M, Morrison JFB, editors: Physiology of the lower urinary tract, London, 1987, Springer-Verlag.
- Elbadawi A: Anatomy and innervations of the vesicourethral muscular unit of micturition. In Krane RJ, Siroky MB, editors: Clinical neuro-urology, Boston, 1991, Little, Brown.
- Rosier PFWM: Bladder function in elderly male patients. Thesis presented to the Department of Urology, School of Medicine, University of Nijmegen, The Netherlands, 1996.
- de Groat WC, Fraser MO, Yoshiyama M, et al: Neural control of the urethra, Scand J Urol Nephrol Suppl 207:35-43, 2001.
- Zderic SA, Chacko S, DiSanto ME, Wein AJ: Voiding function: relevant anatomy, physiology, pharmacology and molecular aspects. In: Gillenwater JY, Grayhack JT, Howards SS, Mitchell ME, editors: Adult and pediatric urology, ed 4, Philadelphia, 2002, Williams & Wilkins, pp 1061-1113.
- Weiss RM: Physiology and pharmacology of the renal pelvis and ureter. In Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors: Campbell’s urology, ed 7, Philadelphia, 1998, WB Saunders.
- Baker JC, Mitteness LS: Nocturia in the elderly, Gerontologist 28:99-104, 1988.
- Brading AF, Mostwin JL: Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation, Br J Pharmacol 98 (4):1083-1090, 1989.
- Parekh AB, Brading AF, Tomita T: Studies of longitudinal tissue impedance in various smooth muscles, Prog Clin Biol Res 327:375-378, 1990.
- DeLancey JO: Functional anatomy of the female pelvis. In Kursh ED, McGuire EJ, editors: Female urology, Philadelphia, 1994, JB Lippincott.
- Brooks JD: Anatomy of the lower urinary tract and male genitalia. In Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors: Campbell’s urology, ed 7, Philadelphia, 1998, WB Saunders.
- Wahle GR, Young GPH, Raz S: Anatomy and physiology of pelvic support. In Raz S, editor: Female urology, Philadelphia, 1996, WB Saunders.
- Redman JF: Anatomy of the genitourinary system. In Gillenwater JY, Grayhack JT, Howards SS, Dukett JW, editors: Adult and pediatric urology, St. Louis, 1996, Mosby.
- Siroky MB: Electromyography of the perineal striated muscles. In Krane RJ, Siroky MB, editors: Clinical neurology, Boston, 1991, Little, Brown.
- Couillard DR, Webster GD: Detrusor instability, Urol Clin North Am 22(3):593-612, 1995.
- Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder, Urology 60(5 suppl 1):7-12, 2002.
- Abrams RM, Stanley H, Carter R, Notelovitz M: Effect of conjugated estrogen on vaginal blood flow in surgically menopausal women, Am J Obstet Gynecol 143:375, 1985.
- Gray M: Urodynamic evaluaton of detrusor instability. Doctoral dissertation, University of Florida, Gainesville, Fla, 1990.
- Zinner NR: Clinical aspects of detrusor instability and the value of urodynamics, Eur Urol 34(suppl 1): 16-19, 1998.
- Gillespie JI, Harvey IJ, Drake MJ: Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity, Exp Physiol 88(3):343-357, 2003.
- Klausner AP, Steers WD: Research frontiers in the treatment of urinary incontinence, Clin Obstet Gynecol 47(1):104-113, 2004.
- Andrew J, Nathan PW, Spanos NC: Cerebral cortex and micturition, Proc R Soc Med 58:533, 1964.
- Athwal BS, Berkley KJ, Hussain I, et al: Brain responses to changes in bladder volume and urge to void in healthy men, Brain 124(2):369-377, 2001.
- Blok BF, Sturms LM, Holstage G: Brain activation during micturition in women, Brain 121(11):2033-2042, 1998.
- Matsuura S, Kakizaki H, Mitsui T, et al: Human brain region response to distention or cold stimulation of the bladder: a position emission tomography study, J Urol 168(5):2035-2039, 2002.
- Nour S, Svarer C, Kristensen JK, et al: Cerebral activation during micturition in normal men, Brain 123(4):781-789, 2000.
