Eyeblink Classical Conditioning
Eyeblink classical conditioning is an associative learning paradigm in which a neutral stimulus such as a tone is presented half a second before a reflex-eliciting stimulus such as a corneal air puff.
Repeated presentation of the tone and air puff cause an association to form, and the organism blinks to the tone before the onset of the air puff. The learned response, which is the new association, is called the conditioned response.
Eyeblink conditioning reveals natural age-related deficits in several nonhuman mammals in which older organisms have been tested — mice, rats, rabbits, cats, and the similarities between age differences in eyeblink conditioning in these animal species and humans are striking.
The processes of normal aging affect eyeblink classical conditioning similarly in all the species.
Among the significant advantage of this model system for gerontology are:
- age differences in the classically conditioned eyeblink responses are large;
- striking parallels exist between the age differences in eyeblink conditioning in nonhuman mammals and humans; and
- the neural circuitry is delineated.
Behavioral and neurobiological parallels have been documented that generalize in aging to all mammals studied, including humans. Moreover, eyeblink conditioning is impaired profoundly in patients with Alzheimer’s disease (AD) Opens in new window, making the procedure relevant for preclinical studies of cognition-enhancing drugs.
In addition to parallels with human behavior and neurobiology, the model system of eyeblink classical conditioning possesses a considerable advantage over the behavioral models commonly used preclinically: the essential neural circuitry in the cerebellum has been identified along with modulatory circuits in hippocampus and cortex.
Delay and Trace Eyeblink Classical Conditioning Procedures
Eyeblink classical conditioning paradigms that have received the greatest research attention are the delay and trace procedures (Figure X-1).
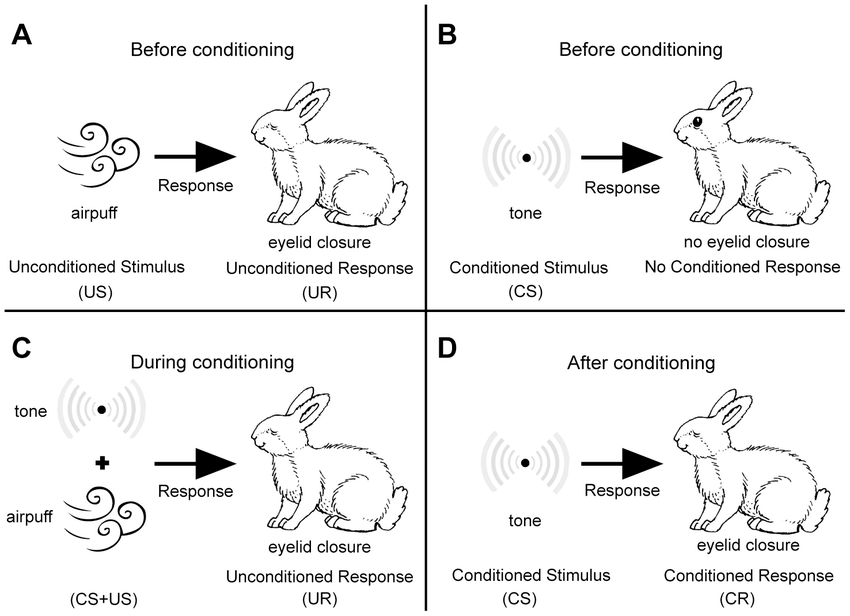 Figure X-1 | A schematic illustration of eye-blink conditioning. Source: ResearchGate Opens in new window
Figure X-1 | A schematic illustration of eye-blink conditioning. Source: ResearchGate Opens in new window |
| (A) Naïve animal responds to the presentation of an airpuff (the US) by eyelid closure. (B) By contrast, a tone (the CS) does not elicit any overt response. (C) During conditioning the CS and the US are repeatedly paired. (D) After conditioning the animal responds to the CS with eyelid closure (the CR). |
In the delay procedure, a neutral stimulus such as a tone conditioned stimulus (CS) is presented before the onset of a corneal air puff unconditioned stimulus (US). The organism learns to blink to the tone conditioned stimulus (CS) before the onset of the air puff unconditioned stimulus (US), and the learned response is called the conditioned response (CR).
The interval between the onset of the conditioned stimulus (CS) and the onset of the unconditioned stimulus (US) is called the interstimulus interval (ISI). The length of this interval affects the rate of conditioning, that is, ISIs greater than 500 ms increase the difficulty level of for rabbits and mice.
In the trace procedure, the conditioned stimulus (CS) is presented and then turned off, and a blank period (trace) ensues before the onset of the unconditioned stimulus (US).
The trace procedure is called hippocampus dependent because organisms with bilateral hippocampal lesions do not acquire conditioned responses (CRs).
- In rabbits, the hippocampus is essential when the trace interval exceeds 300 ms.
- In humans, the trace interval must be 1000 ms for eyeblink conditioning to be abolished by bilateral hippocampal lesions.
- In mice, bilateral ibotinic acid lesions of hippocampus abolish eyeblink conditioning in a 500-ms trace procedure with a trace interval of 250 ms.
The cerebellar interpositus nucleus is essential in all eyeblink conditioning procedures.
Brain Circuits and Associative Learning
- Cerebellum
A variety of techniques have been used to demonstrate that the dorsolateral interpositus nucleus ipsilateral to the conditioned eye is the essential site for acquisition and retention of conditioned eyeblink responses. The techniques include:
- electrophysiological recording of multiple and single units,
- electrolytic and chemical lesions,
- physical and chemical reversible lesions,
- neural stimulation,
- genetic mutations, and
- pharmacological manipulation.
Selective lesions (electrolytic or chemical) of the cerebellum prevent the acquisition and retention of conditioned eyeblink responses.
Electrophysiological recording of single and multiple-unit activity in the cerebellum indicated that cells in specific cerebellar regions undergo learning-induced changes during eyeblink conditioning.
The involvement of the cerebellum in eyeblink conditioning is also supported by studies that show that electrical stimulation of the two major afferents to the cerebellum — the mossy fibers from the pontine nuclei and the climbing fibers from the inferior olive — can substitute for the externally presented conditioned and unconditioned stimuli and can lead to learning.
Reversible inactivation of the anterior interpositus nucleus and overlying cerebellar cortex during training completely prevents learning, but when the nucleus is returned to its normal functional status, learning occurs.
Inactivation of the efferent output of the interpositus nucleus (i.e., in the superior cerebellar peduncle and red nucleus) does not prevent learning.
A model of the neural circuitry essential for eyeblink conditioning indicates that both the cerebellar cortex and the interpositus nucleus receive information about the conditioned stimulus (CS; that can be a tone or light), conveyed by the mossy fiber system emanating from the pontine nucleus, and information about the unconditioned stimulus (US; i.e., a puff of air to the cornea or a shock to the eye muscles), relayed by the climbing fiber system originating from the inferior olive.
Converging signals from the conditioned and unconditioned stimuli are relayed to Purkinje cells in cerebellar cortex and principal cells in interpositus nucleus.
The efferent (eyelid closure) conditioned responses pathway projects from the interpositus nucleus in cerebellum to the red nucleus and via the descending rubral pathway to act ultimately on motor neurons.
A cellular model system proposed as a mechanism for information storage in the cerebellum is long-term depression (LTD).
In this model, coactivation of climbing fiber and parallel fiber inputs to a Purkinje cell induces a persistent, input-specific depression of the parallel fiber-Purkinje cell synapse.
Studies using mutant and transgenic mice with alterations in cerebellar cortex that affected LTD demonstrated a consistent correlation between impaired cerebellar cortical LTD and impaired eyeblink conditioning.
- Hippocampus
The hippocampus Opens in new window and the septohippocampal acetylcholine system are normally engaged in basic associative learning of the sort represented by delay eyeblink classical conditioning and the hippocampus is essential in trace eyeblink conditioning.
In a series of studies using delay eyeblink classical conditioning in rabbits, it was observed that activity recorded in the CA1 pyramidal cell region of the hippocampus forms a predictive ‘model’ of the amplitude-time course of the learned behavioral response, but only under conditions where behavioral learning occurs.
This response is generated largely by pyramidal neurons.
The role of the hippocampus in delay eyeblink classical conditioning is called ‘modulatory’ because manipulations of the hippocampus can impair or enhance the rate of acquisition.
The memory trace itself is not in the hippocampus, but the hippocampus can markedly influence the storage process. Although the hippocampus is not necessary for normal acquisition in the delay procedure, it is necessary for trace conditioning.
There is a long-lasting neuronal plasticity formed in the hippocampus following eyeblink conditioning. This change is essential for learning to occur in the trace eyeblink conditioning procedure, at least until the learning is consolidated.
Hippocampal slices prepared from animals trained previously in trace eyeblink conditioning had pyramidal neurons showing a marked reduction in the slow afterhyperpolarization compared to neurons in slices from control animals that received presentations of tones and air puffs that were not related.
Normal Aging and Eyeblink Classical Conditioning
Since the first studies were carried out in humans in the 1950s comparing young and older adults on delay eyeblink classical conditioning, striking age differences were apparent.
When adults over the age range of 20–90 are tested, age-related effects appear in the decade of the 40s. direct comparisons of a hippocampus-dependent memory measure (California Verbal Learning Test) and a cerebellum-dependent measure (400 ms delay eyeblink classical conditioning) in the same young and older adults revealed a larger age effect on the cerebellum-dependent than the hippocampus-dependent task.
These results are consistent with the studies of neural aging that demonstrate significant loss of Purkinje neurons relatively early in the adult life span and age-related stability in hippocampal neuron numbers, with altered hippocampal electrophysiology demonstrated in late life in nonhuman mammals.
Similar to all investigations of normal aging, when participants have subclinical pathology as in the early stages of Alzheimer’s disease (AD) Opens in new window, performance is impaired significantly more than it is affected by processes of aging.
Indeed, several laboratories have demonstrated that patients diagnosed with probable AD are severely impaired on this task.
Disruption of the septohippocampal acetylcholine neurotransmitter system impairs acquisition of eyeblink conditioning system is dramatically impaired, such that this dysregulation is the likely cause of severe impairment of delay eyeblink classical conditioning in AD.
Effects of Aging on Eyeblink Classical Conditioning and Relation to Neural Changes
A strength of the eyeblink conditioning model for research on aging is that humans and nonhuman mammals show similar age-related deficits.
Humans begin to show age-associated deficits in eyeblink conditioning between 40 and 50 years, while rabbits begin to show age-associated deficits at round 2 years.
Based on declines in reproductive capacity, a 2-year-old rabbit is equivalent to a 35–40-year-old human, which suggests similar onset of age-associated declines in eyeblink conditioning in both species.
Results with aging in humans and rabbits generalize to other nonhuman species, such as rats, cats, and mice.
Aged mice are impaired in middle age (9–12 months) in 250-ms delay and at an older age (18–24 months) in 500-ms delay eyeblink conditioning just as humans are impaired in middle age (45-55 years) in 400-ms delay and at an older age (70–80 years) in 500-ms delay eyeblink conditioning.
Loss of Purkinje neurons in cerebellar cortex is associated with age-related deficits in eyeblink conditioning in several species rabbits and mice with direct Purkinje cell counts and humans with indirect measure, MRI-assessed cerebellar volume.
With regard to the hippocampus, afterhyperpolarization recorded in slice preparations from trained rabbits was observed in relation to normal aging.
Excitability of CA1 neurons was studied 24 h after the last training session in aged rabbits that reached a 60% behavioral criterion (learning-intact), rabbits trained for 30 days that never demonstrated more than 30% CRs per session (failed to learn), and naïve aging rabbits.
Aged CA1 neurons from learning-intact animals had significantly reduced postburst afterhyperpolarizations and reduced spike-frequency adaptation compared with neurons from control groups of naïve and aging rabbits that failed to learn.
See also:
- Cartford MC, Gould T, and Bickford PC (2004) A central role for norepinephrine in the modulation of cerebellar learning tasks. Behavioral and Cognitive Neuroscience Reviews 3: 131–138.
- Chawla MK and Barnes CA (2007) Hippocampal granule cells in normal aging: Insights from electrophysiological and functional imaging experiments. Progress in Brain Research 163: 661-678.
- Christian KM and Thompson RF (2003) Neural substrates of eyeblink conditioning: Acquisition and retention. Learning and Memory 11: 427–455.
- Disterhoft JF and Oh MM (2007) Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 6: 327&ndaash;336.
- Finch CE and Kirkwood TBL (2000) Chance, Development, and Aging. New York: Oxford University Press.
- McEchron MD, Weible AP, and Disterhoft JF (2001) Aging and learning specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. Journal of Neurophysiology 86: 1839-1857.
- Thibault O, Gant JC, and Landfield PW (2007) Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: Minding the store, Aging Cell 6: 307–317.
- West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus, Neurobiology of Aging 14: 287-293.
- Woodruff-Pak DS, Lehr MA, Li J-G, and Liu-Chen, L-Y (in press) Good learners have higher levels of brain nicotinic receptor binding than poor learners. Neurobiology of Aging.

