Menstrual Cycle Physiology
Anatomy of the Female Sex Organs and Physiology of the Menstrual Cycle
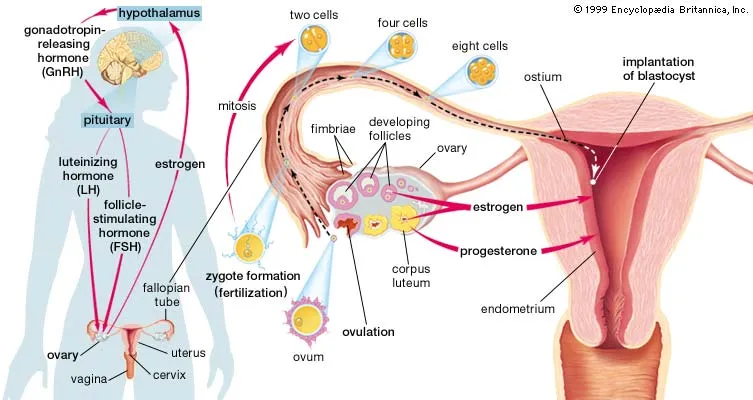 Figure X-1. The female reproductive unit | Credit: BritannicaOpens in new window
Figure X-1. The female reproductive unit | Credit: BritannicaOpens in new windowThe female reproductive system is a multiorgan system comprised of the hypothalamus, pituitary gland, ovaries, uterus, and vagina. |
Pertinent anatomy involved with the menstrual cycle includes the ovaries, a paired organ which has both a reproductive and an endocrine function. This is where eggs are formed and female sex hormones are reproduced.
Three levels of hormones are normally secreted in a feedback loop termed the hypothalamic-pituitary-ovarian (HPO) axis:
- by the hypothalamus — gonadotropin-releasing hormone (GnRH);
- by the anterior pituitary — follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in response to GnRH; and
- by the ovaries — two gonadal steroids, estrogen and progesterone, in response to FSH and LH.
Regulation of the menstrual cycle is controlled by both LH and FSH from the anterior pituitary gland in response to GnRH from the hypothalamus. These hormones are secreted in different amounts during various phases of the monthly menstrual cycle.
The onset of menarche in females signals the transition from childhood to the pubertal, reproductive state, correlating with both body height and bone maturation.
MenarcheOpens in new window normally occurs about two years after the appearance of secondary sex characteristicsOpens in new window (breast and pubic hair development) around age 11 – 13 years, but can begin as early as 7 years old. Menarche is relatively a late determinant of pubertal development.
If menstruationOpens in new window does not occur by age 16, this delay of menarche (primary amenorrheaOpens in new window) may be due to excessive exercise or insufficient intake of appropriate nutrition, among other factors.
The menstrual cycle occurs on a regular basis between 20 and 45 days, on average approximately every 4 weeks (28 days), with menstrual flow lasting from 3 to 7 days.
Typically, three separate phases encompass the normal menstrual cycle:
- follicular or proliferative phase (growth of the endometrium or uterine lining stimulated by estrogen),
- ovulatory phase (ovulation or egg release in response to LH), and
- luteal or secretory phase (transformation of the endometrium from a proliferating into secreting-type tissue under progesterone influence).
The duration of the follicular phase is more variable between different women, whereas the luteal phase is consistently 14 days. The first day of the menstrual cycle is marked with the initial sign of vaginal bleeding.
The onset of the follicular phase is stimulated by FSH more so than LH; the quantity of LH slowly increases during the follicular phase until peaking levels at mid-cycle.
High amounts of FSH during the beginning of the luteal phase stimulate growth of several ovarian follicles then levels begin to decline until late luteal phase. During this time both progesterone and estrogen serum levels are low. Estrogen then increases steadily, peaking just before ovulation, generally occurring on the same day of the LH surge.
This rise in estrogen occurs about the middle of the cycle, day 14, when one follicle is released in the ovulation phase.
The luteal phase then takes over during the second half of the cycle under the continuing influence of LH. Progesterone then increases above estrogen levels, though the latter hormone concentration remains fairly high.
These two hormones, estrogen and progesterone, are secreted by the corpus luteum which then degenerates, causing both hormonal levels to concurrently fall as long as the egg is neither fertilized nor implanted in the uterus. The monthly cycle then repeats itself by shedding of the endometrium, manifested by menstrual flow, to restart the follicular phase all over again under FSH influence (Figure X-2).
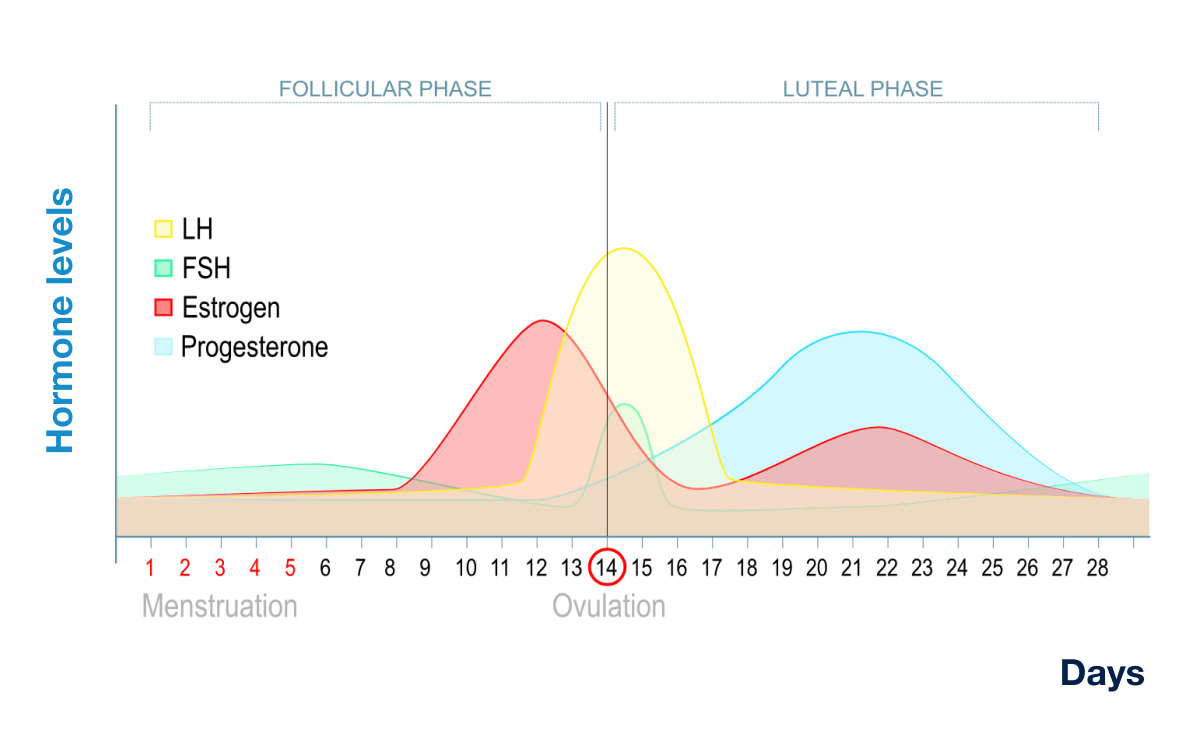 Figure X-2. Menstrual cycle. FSH [follicle stimulating hormone], LH [lutenizing hormone]. | Credit University of California, Center for Reproductive Health.Opens in new window
Figure X-2. Menstrual cycle. FSH [follicle stimulating hormone], LH [lutenizing hormone]. | Credit University of California, Center for Reproductive Health.Opens in new window
|
Effects of Female Sex Hormones on Bone
The female reproductive system plays a major role in the maintenance of the skeletal bone integrity from menarche to menopause. The skeleton is one of the main target tissues for estrogen, and to a lesser extent, progesterone. Bone cells contain estrogen and progesterone receptors that positively stimulate bone formation and suppress bone resorption. Thus, they are key regulators of bone turnover, adjusting the bone mass “set point” by keeping in balance the quantity of bone being formed with the amount that is reabsorbed (coupling effect).
Sex hormone receptors have been found in growth plate chondrocytes (cartilage cells) during puberty. At the early stages of puberty, sex hormones stimulate longitudinal bone lengthening of the diaphyses (shafts of long bones) resulting in the “pubertal growth spurt”.
Estrogen acts directly through inhibiting bone resorption by osteoclasts while increasing the activity of bone forming cells, osteoblasts. However, at the late stages of puberty the sex-specific hormones, primarily estrogen, play a large role in the closure of growth plates. Estrogen, therefore, highly influences the final height achieved in femals.
Estrogen and progesterone not only play an essential role on homeostasis of the skeleton by exerting their direct effects through receptors on bone cells but also exert indirect effects on other systemic hormones.
These sex hormones work in conjunction with other hormones and steroids to further facilitate bone development. For example, estrogen regulates circulating levels of growth hormone (GH) and insulin growth-like factor (IGF-1); these two hormones stimulate longitudinal bone growth.
In a cross-sectional study conducted on children, serum IFG-1 levels were positively associated with bone growth. Insuling growth-like factor receptors (IGF-1R) are also located in the kidneys, where they are associated with the production of the hormonal form of vitamin D.
Environmental factors can also stimulate bone formation. Mechanical loading of bone exerts a positive effect on bone formation at points of stress, in a process defined by Wolff’s Law. A cross-sectional study found hip and spine BMD values 30 – 40 % greater in female gymnasts compared to long-distance runners. This implies that the higher impact forces generated in gymnastics (10 – 12 times body weight) as compared to running (3 – 5 times body weight) positively affect BMD. Bone formation can also be altered by external agents such as certain metals, pesticides, and components in cigarette smoke).
To summarize, the normal, monthly occurrence of the female menstrual cycle represents an intricate interaction of three organs – hypothalamus, anterior pituitary, and ovaries – resulting in a feedback mechanism whereby several secreted hormones influence each other to effect maturation and ovulation of an egg (follicle).
Next, the endometrium becomes prepared to receive the mature follicle and, if no implantation occurs, then sloughing of the uterine lining manifests itself by menstrual bleeding and the cycle repeats.
Disturbances in any portion of this hormonal loop between the reproductive system and these two specific centers in the brain could, in turn, alter the quality/quantity of menstruation and secondarily affect bone deposition, especially during the critical time of initial peak bone mass acquisition involved with the pubertal/adolescent stage.
See also:
- AmenorrheaOpens in new window
- DysmenorrheaOpens in new window
- FHAOpens in new window
- Menstrual Cycle Physiology & Anatomy of the Female Sex OrgansOpens in new window
- Menstrual Cycle Effects on Peak Bone MassOpens in new window
- Premenstrual Syndrome (PMS)Opens in new window
- Premenstrual Dysphoric Disorder (PMDD)Opens in new window
- EndometriosisOpens in new window

