Sleep
Sleep Enhances Declarative Memories
The notion that sleep Opens in new window enhances memories Opens in new window began in the early 1900s. In one early study very reminiscent of methods still currently used in the field, G.E. Muller’s student Rosa Heine (1914) had four subjects study lists of nonsense syllables and tested their memory for these syllables after a 24-hour delay. The key aspect of her method was that learning of the lists could take place either before a night of sleep or before a day of wake. Thus, learning the syllables could be followed very closely by nocturnal sleep and then a day of wake, or could be followed by many hours of wake and then a night of sleep. The difference between the two conditions was how long after learning the subject went to sleep.
Heine found that learning the lists just before sleep resulted in significantly better memory of the syllables during the test than when learning occurred before a period of wake. Although these pointed toward the critical impact of sleep soon after learning, it was generally concluded at the time that sleep was merely shielding the individual from “the interference, inhibition, or obliteration of the old by new” (Jenkins and Dallenbach, 1924, p. 612; see Van Ormer, 1933 for a review).
This view was held even despite the fact that Heine’s study allowed both groups to receive approximately equal amounts of waking time and thus should have equated interference across the groups. However, research on this topic later expanded and matured, resulting in an extended understanding of the cognitive and neural mechanism acting on declarative memories during sleep and leading to a wealth of literature on the matter.
These recent studies have incorporated safeguards for the methodological problems of their predecessors. For instance, while Heine’s (1914) work investigated performance of only a handful of subjects, did not control for circadian influences, and did not regulate testing times for all subjects within the same group, current studies have consistently increased sample sizes, utilized control groups, and standardized data collection methods.
Sleep and Memory for Verbal Stimuli
Like Heine’s (1914) study, which used nonsense syllables, modern sleep studies have also investigated memory for verbal stimuli. However, instead of nonsense syllables, current studies commonly test memory for words, or pairs of words. Such studies generally show that sleep enhances memory for words or word pairs more so than an equivalent time spent awake (Lahl et al., 2008; Payne et al., 2012; Plihal and Born, 1997; Tuck et al., 2006), and this effect has been replicated using various methodologies.
In one of these studies, Plihal and Born (1997) investigated whether specific sleep stages might be particularly important for memory improvements in word-pair learning. They required participants to learn pairs of words, and immediately tested them on these pairs by giving them the first word and asking them to fill in the missing word of the pair. Participants were tested on these pairs until they could remember 60% of the pairs. Following this immediate test, they underwent three hours of sleep or three hours of wake, and were then retested.
Most important to their design, this three-hour interval could occur over either the first three hours of nocturnal sleep or the last three hours. In the first case, termed “early” sleep, participants learned the task prior to bedtime and were awoken after three hours of sleep for testing. In the latter case, termed “late” sleep, participants slept for three hours, were awoken to learn the task, and then received another three hours of sleep before being awoken and tested for the final time. Such a design is often employed to better separate the effects of the different types of sleep on such tasks.
As mentioned, the first half of the night is dominated by SWS, while the second half of the night contains more REM, thus allowing researchers to better investigate the contributions of these specific stages to performance. The authors found that those who underwent three hours of early sleep improved their recall of the pairs above the immediate test performance significantly more than those who underwent three hours of wake or three hours of late sleep, suggesting that SWS played an important role in performance improvements. This effect has also been found with a nap paradigm. In such a model, participants are compared by the presence or absence of a nap, usually during the early afternoon and lasting less than an hour.
Tucker and colleagues (2006) found that a short nap of about 47 minutes during a six-hour interval between learning and recall testing of a list of word pairs significantly improved memory relative to baseline testing. This effect was not seen in those who did not nap during this interval. Importantly, such a short nap prevented participants from obtaining any sign of REM sleep, restricting the sleep associated with these improvements only to NREM stages, a finding consistent with that of Plihal and Born (1997).
However, it is interesting that many studies of this type fail to find significant correlations between performance and specific sleep stages. Tucker et al. (2006) did not obtain such a relationship, nor did Lahl and colleagues (2008), who reproduced the sleep-dependent memory effect for words with a 25– and even a 6-minute nap. They did, however, find that the amount of time it took to fall sleep impacted the performance enhancement afforded by sleep, such that those who fell asleep faster benefited more from the shorter naps. Such a finding suggests that getting at least some amount of sleep after learning, especially directly after learning, is sufficient for memory improvements.
However, similar to problems faced by researchers during Heine’s time, some critics still argue that sleep could simply be protecting memories from interfering stimuli, since there is relatively little processing of outside information during sleep (see Ellenbogen, Payne, and Stickgold, 2006; Wixted, 2004, for difference opinions on the matter).
Recent studies have challenged this assumption by manipulating characteristics of the learned material, and altering the way in which sleep impacts memory. For instance, Payne et al., (2012) required participants to study pairs of words that were either semantically related or unrelated before a 12-hour delay that contained either sleep or wake. They found that participants who slept or stayed awake during the delay recalled the lists containing related words equally well.
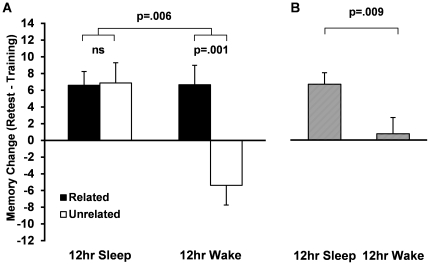 |
| Figure X2 (A) Recall performance for related (black) and unrelated (white) word pairs after a 12-hour consolidation period containing a period of sleep or wake. (B) Recall performance irrespective of word pair type. Source: Payne et al., (2012). |
However, while participants who stayed awake had much more difficulty remembering the lists containing unrelated words, those who slept remembered the pairs on this list just as well as those on the related word lists (Figure X2). If sleep was acting to simply buffer memories from interfering information, it would be expected that the sleep and wake groups would differ on memory for both types of word pairs, not just one.
Payne et al., (2012) also used this paradigm with two other groups of participants. These subjects underwent a 24-hour delay between studying the word pairs and final testing. Thus, participants received both a period of wake and a period of sleep before final testing, but half of participants obtained sleep shortly after the study phase, while the other half studied the words and then slept only after a full day awake.
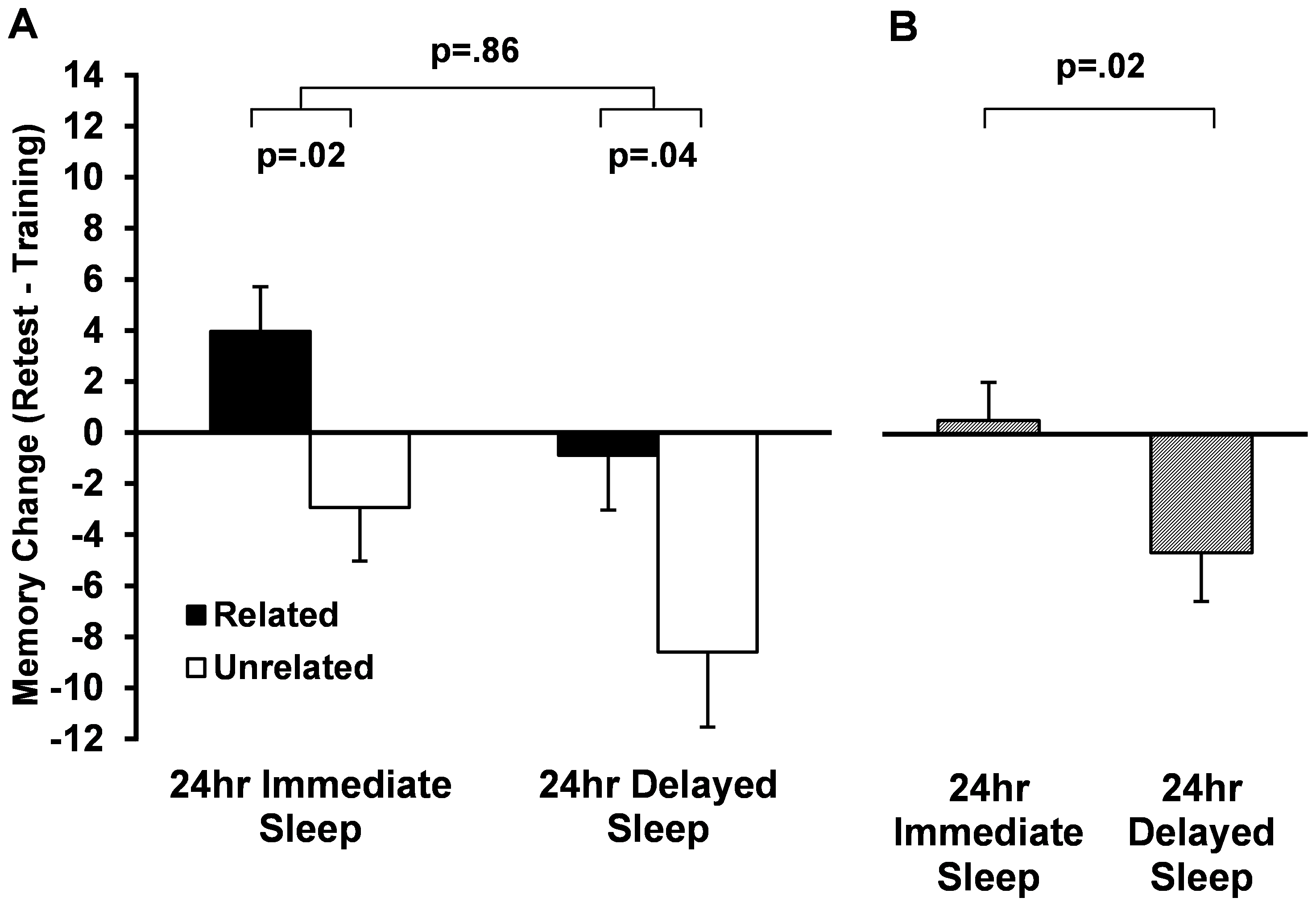 |
| Figure X3 (A) Recall performance for related (black) and unrelated (white) word pairs after a 24-hour consolidation period containing an immediate period of sleep or sleep after a day of wake. (B) Recall performance irrespective of word pair type. Source: Payne et al. (2012). |
Related to performance after only a 30-minute delay and after the 12-hour delays, they found that the number of the unrelated word pairs forgotten over the wake portion of the 24-hour delay was doubled when this portion came before sleep as opposed to following sleep. However, the amount of forgetting occurring over the sleep portion of the 24 hours was the same irrespective of its relationship to the wake portion.
This finding is important because, if sleep were simply shielding against interference, the amount of forgetting over the waking portion of the delay would be the same, regardless of whether wake preceded or followed sleep. Yet forgetting was increased over the wake period when sleep was delayed after learning (Figure X3). This suggests that, rather than protecting against interference, sleep may actually work to stabilize memories, providing the most benefit when it closely follows learning (Payne et al., 2012).
Sleep and memory for picture stimuli
The impact of sleep on the ability to remember picture stimuli has also been a topic of interest in the sleep and memory literature. This line of study commonly employs methods similar to those used to investigate memory for verbal stimuli, and comparable effects of sleep on memory have been obtained between the two classes of stimuli. For example, to examine memory for image-location pairings, Talamini and colleagues (2008) placed pictures of different faces in various locations on a computer screen and had participants learn these face-location pairings. They were tested on these pairings after a brief 10-minute interval, as well as after a 12-hour delay containing either a night of sleep or a day of wake.
They found that those who slept forgot significantly fewer of the pairings than those who remained awake. Similar to methods of Payne and colleagues (2012), two additional groups had a 24-hour delay between the learning and final testing sessions. One group obtained sleep during the first portion of this delay, while the other obtained sleep only after a full day of wake.
 |
| Figure X4 Amount of forgetting occurring across the 12-hour groups containing either a period of wake or a period of sleep, and the 24-hour groups containing either wake before sleep or sleep before wake. Source: Talamini et al. (2008) |
Like Payne et al. (2012), Talamini et al., found that significant forgetting occurred in those subjects who remained awake for an entire day before sleeping, but not for those who slept first (Figure 11.4). Again, given that both groups received the same amount of sleep and wake before testing, a passive role of sleep in preventing interference can be ruled out. Clearly, sleeping soon after learning affords a greater benefit to memory than if sleep is delayed.
Some studies have shown that this positive impact of sleep after learning can have long-term effects on memory, resulting from the reorganization of the memory trace within the brain. For example, Takashima and colleagues (2006) had subjects memorize a set of images depicting large-scale spatial layouts of natural landscapes and then nap for roughly 90 minutes. Participants then memorized a new set of the pictures before taking a recognition test of all the pictures they had studied. They found that the amount of SWS obtained during this nap was positively associated with recognition performance on only those items studied before the nap, suggesting an enhancing effect of this sleep stage on memory consolidation that is consistent with previous findings for verbal stimuli (Plihal and Born, 1997).
Summary
Collectively, these investigations have led to a large body of evidence supporting sleep’s positive effects on the declarative memory system. Improving upon sleep studies from the early 1900s, current studies have found a significant role for sleep in forming memories for previously encountered word pairs, scenes, and locations—all types of memories that are particularly dependent on the hippocampus.
Such information comes from a wide variety of methodologies, including early/late nocturnal sleep paradigms, nap studies, and research focused on extended delays. Such research has led to stronger arguments against an interference account and has targeted SWS as an important stage for declarative memory improvements across all discussed stimuli domains.
However, there may also be a role for the timing of sleep after learning, as delaying sleep too long in humans can lead to decreased performance on both verbal and picture memory tasks, even after sleep is finally achieved (Payne et al., 2012; Talamini et al., 2008). Thus, the amount, timing, and type of sleep are important factors to consider when acquiring a range of new declarative memories.
See also:
Cited references in the book include:
- Barbera, J. (2008). Sleep and dreaming in Greek and Roman philosophy. Sleep Medicine, 9 (8), 906-910. doi: 10.1016/j.sleep.2007.10.010.
- Born, J., and Wilhelm, . (2012). System consolidation of memory during sleep. Psychological Research, 76 (2), 192-203. doi: 10.1007/S00426-011-0335-6.
- Braun, A.R., Balkin, T.J., Wesenten, N.J., et al. (1997). Regional cerebral blood flow throughout the sleep-wake cycle: an H218O PET study. Brain, 120 (7), 1173-1197. doi: 10.1093/brain/120.7.1173
- Carskadon, M.A., and Dement, W.C. (1989). Normal human sleep: an overview. In Principles and Practice of Sleep Medicine (ed. M.H. Kryger, T. Roth, and W.C. Dement). Philadelphia: W.B. Saunders, pp.3-13.
- Corkin, S. (1968). Acquisition of motor skill after bilateral medial temporal lobe excision. Neuropsychologia, 6 (3), 255-265. doi: 10.1016/0028-3932(68)90024-9.
- Dallman, M.F., Bhatnagar, S., and Viau, V. (2000). Hypothalamo-pituitary adrenal axis. In Encyclopedia of Stress (ed. G. Fink). San Diego: Academic Press, pp. 468-477.
- De Gennaro, L., and Ferra, M. (2003). Sleep spindles: an overview. Sleep Medicine Reviews, 7 (5), 423-440. doi: 10.1053/smry.2002.0252.
- de Kloet, E.R., Oitzl, M.S., and Joёls, M. (1999). Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences, 22 (10), 422-426. doi: 10.1016/S0166-2236(99)01438-1.
- Dement, W.C., and Vaughan, C. (2000). The Promise of Sleep: A pioneer in Sleep Medicine Explores the Vital Connection Between Health Happiness and a Good Night’s Sleep. New York, NY: Random House.
- Ellenbogen, J.M., Payne, J.D., and Stickgold, R.R. (2006). The role of sleep in declarative memory consolidation: passive and permissive, active or none? Current Opinion in Neurobiology, 16 (6), 716-722. doi: 10.1016/j.conb.2006.10.006.
- Fisher, S., Nitschke, M.F., Melchert, U.H., et al. (2005). Motor memory consolidation in sleep shapes more effective neuronal representations. Journal of Neuroscience, 25 (49), 11248-11255. doi: 10.1523/JNEUROSCI.1743-05.2005.
- Fogel, S.M., and Smith, C.T. (2006). Learning-dependent changes in sleep spindles and stage 2sleep. JournalofSleepResearch, 15 (3), 250-255. doi:10.1111/j.1365-2869.2006.00522.x.
- Fogel, S.M., and Smith, C.T. (2011). The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience and Behavioral Reviews, 35 (5), 1154-1165. doi:10.1016/j.neurbiorev.2010.12.003.
- Fogel, S.M., Smith, C.T., and Cote, K.A. (2007). Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behavioral Brain Research, 180 (1), 48-61. doi: 10.1016/j.bbr.2007.02.037.
- Fries, E., Dettenborn, L., and Kirschbaum, C. (2009). The cortisol awakening response (CAR): facts and future directions. International journal of Psychophysiology, 72 (1), 67-73. doi: 10.1016/j.ijpsycho.2008.03.014.
- Gais, S., and Born, J. (2004). Declarative memory consolidation: mechanisms acting during human sleep. Learning and Memory, 11 (6), 679-685. doi: 10.1101/lm.80504.
- Gais, S., Mölle, M., Helms, K., and Born, J. (2002). Learning-dependent increases in sleep spindle density. Journal of Neuroscience, 22 (15), 6830-6834. doi: 0270-6474/02/226830-05$15.00/0.
- Gais, S., Plihal, W., Wagner, U., and Born, J. (2000). Early sleep triggers memory for early visual discrimination skills. Nature Neuroscience, 3 (12), 1335-1339.
- Giuditta, A., Ambrosini, M.V., Montagnese, P., et al. (1995). The sequential hypothesis of the function of sleep. Behavioral Brain Research, 69 (1), 157-166. doi: 10.1016/0166-4328(95)00012-I.
- Hamann, S. (2001). Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences, 5 (9), 394-400. doi: 10.1016/S1364-6613(00)01707-1.
- Hamann, S.B., Ely, T.D., Hoffman, J.M., and Kilts, C.D. (2002). Ectasy and agony: activation of human amygdala in positive and negative emotion. Psychological Science, 13 (2), 135-141. doi:10.1111/1467-9280.00425.
- Walker, M.P., Brakefield, T., Morgan, A., et al. (2002). Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron, 35 (1), 205-211. doi: 10.1016/S0896-6273(02)00746-8.
- Walker, M.P., Brakefield, T., Seidman, J., et al. (2003). Sleep and the time course of motor skill learning. Learning and Memory, 10 (4), 275-284. doi:10.1101/lm.58503.
- Walker, M.P., and Stickgold, R. (2006). Sleep, memory, and plasticity. Annual Review of Psychology, 57, 139-166. doi: 10.1146/annurev.psych.56.091103.070307.
- Wixted, J.T. (2004). The psychology and neuroscience of forgetting. Annual Review of Psychology, 55, 235-269. doi: 10.1146/annurev.psych.55.090902.141555.

