Autosomal Dominant Inheritance
Transmission Patterns in Disorders of Autosomal Dominant Inheritance
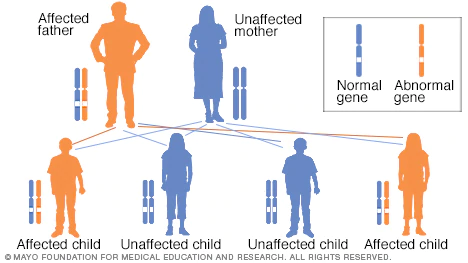 Image courtesy of MAYO CLINIC Opens in new window
Image courtesy of MAYO CLINIC Opens in new windowDisorders of autosomal dominant inheritance usually exhibits a vertical pattern of transmission, meaning that the phenotype usually appears in every generation, with each affected person having an affected parent. For each offspring of an affected parent, the risk of inheriting the mutated allele is 50%.
Clinical Features
Disorders of autosomal dominant inheritance are manifested in the heterozygous state. Therefore at least one parent in an index case usually is affected; both males and females are affected, and both can transmit the disease.
When an affected person engage marries an unaffected one, every child has one chance in two of having the disease. The following features also pertain to autosomal dominant diseases:
- With any autosomal dominant disorder, some patients do not have affected parents. Such patients owe their disorder to new mutations involving either the egg or the sperm from which they were derived. Their siblings are neither affected nor at increased risk for development of the disease.
- Clinical features can be modified by reduced penetrance and variable expressivity. Some persons inherit the mutant gene but are phenotypically normal. This mode of expression is called reduced penetrance. The variables that affect penetrance are not clearly understood. In contrast with penetrance, if a trait is consistently associated with a mutant gene but is expressed differenty among persons carrying the gene, the phenomenon is called variable expressivity. For example, manifestations of neurofibromatosis 1 range from brownish spots on the skin to multiple tumors and skeletal deformities.
- In many conditions, the age at onset is delayed, and symptoms and signs do not appear until adulthood (as in Huntington disease).
- In autosomal dominant disorders, a 50% reduction in the normal gene product is associated with clinical signs and symptoms. Because a 50% loss of enzymes activity can be compensated for, involved genes in autosomal dominant disorders usually do not encode enzyme proteins, but instead fall into two other categories of proteins: (1) those involved in regulation of complex metabolic pathways, (2) key structural proteins, such as collagen and cytoskeletal components of the red cell membrane.
Disorders of autosomal dominant inheritance often involve mutations in genes that regulate complex metabolic pathways or produce structural proteins. Examples of autosomal dominant disorders include Huntington disease Opens in new window (triplet nucleotide repeats), osteogenesis imperfect (mutations in the collagen gene), and familial hypercholesterolemia (mutations in the receptor for very-low-density lipoproteins).
Whenever the gene Opens in new window is present, it is expressed in the phenotype Opens in new window and can be traced through a number of generations. Expression of these genes rarely skips a generation, and a person not affected will not transmit the gene.
Therefore the affected individual will have an affected parent, unless the condition is the result of fresh mutation, which is a common finding in most autosomal dominant conditions. An exception to this is Huntington disease Opens in new window, in which new mutations are extremely rare.
Incidence
The incidence of some autosomal dominant disorders is high, at least in specific geographical areas: for example, 1 in 500 for familial hypercholesterolemia Opens in new window in populations of European or Japanese descent; 1 in 550 for myotonic dystrophy in the Charlevoix and Saguenay-Lac Saint Jean regions in northeaster Quebec; and about 1 in 2500 to 3000 for several conditions, such as Huntington disease Opens in new window in populations of northern European origin, neurofibromatosis, and polycystic kidney disease.
Although many autosomal dominant disorders are individually much less common, they are so numerous in the aggregate that their hereditary nature; when they are transmitted through families, they become problems not only for individuals but also for whole kindreds, often through many generations. In some cases, the burden is compounded by social difficulties resulting from physical or mental disability.
The risk and severity of dominantly inherited disease in the offspring depend on whether one or both parents are affected and whether the trait is strictly dominant or incompletely dominant.
Denoting “D” as the mutant allele and “d” as the normal allele, matings that produce children with an autosomal dominant disease can be between two heterozygotes (“D/d”) for the mutation or, more frequently, between a heterozygote for the mutation (“D/d”) and a homozygote for a normal allele (“d/d”):
| Parental Mating | Offspring | Risk to Offspring |
|---|---|---|
| Affected by unaffected “D/d” x “d/d” | 1/2 “D/d”, 1/2 “d/d” | 1/2 affected 1/2 unaffected |
| Affected by affected “D/d” x “D/d” | 1/4 “D/D”, 1/2 “D/d”, 1/4 “d/d” | If strictly dominant: 3/4 affected 1/4 unaffected If incompletely dominant: 1/2 affected similarly to the parents 1/4 affected more severely than the parents 1/4 unaffected |
Each child of “D/d” by “d/d” mating has a 50% chance of receiving the affected parent’s abnormal allele “D” and a 50% chance of receiving the normal allele “d”.
In the population as a whole, the offspring of “D/d” by “d/d” parents are approximately 50% “D/d” and 50% “d/d”. Of course, each pregnancy is an independent event, not governed by the outcome of previous pregnancies. Thus, within a family, the distribution of affected and unaffected children may be quite different from the theoretical expected ratio of 1:1, especially if the sibship is small.
Typical autosomal dominant inheritance can be seen in the pedigree Opens in new window of a family with a dominantly inherited form of hereditary deafness. In medical practice, homozygotes for dominant phenotypes are not often seen because matings that could produce homozygous offspring are rare.
Again denoting the mutant allele as “D” and the normal allele as “d”, the matings that can produce a “D/D” homozygote might theoretically be “D/d” by “D/d”, “D/D” by “D/d”, or “D/D” by “D/D”, or the patient might, in exceedingly rare instances, have received a new mutation from a genetically unaffected parent.
Practically speaking, however, only the mating of two heterozygotes need be considered because “D/D” homozygotes are very rare and generally too severely affected to reproduce (fitness = 0).
In the case of two heterozygotes matings, 3/4 of the offspring of a “D/d” by “D/d” mating will be affected to some extent and 1/4 unaffected. In theory, the 3/4 affected could all have the same condition if it is a pure dominant, or 1/3 of the affected would be homozygotes and much more severely affected than the “D/d” heterozygotes if it is an incompletely dominant condition.
In fact, as earlier said, no dominant human disorders have been clearly proved to be pure dominants. Even Huntington disease Opens in new window, which is the disorder most frequently claimed to be a pure dominant because the disease is generally similar in the nature and severity of symptoms in heterozygotes and homozygotes, appears to have a somewhat accelerated time course from the onset of disease to death in homozygous individuals compared with heterogygotes.
See also:
- Adapted from Thompson & Thompson Genetics in Medicine E-Book By Robert L. Nussbaum, Roderick R. McInnes, Huntington F Willard

